Please use a PC Browser to access Register-Tadawul
FDA NDA Move Puts Exelixis Colorectal Cancer Bet To The Test
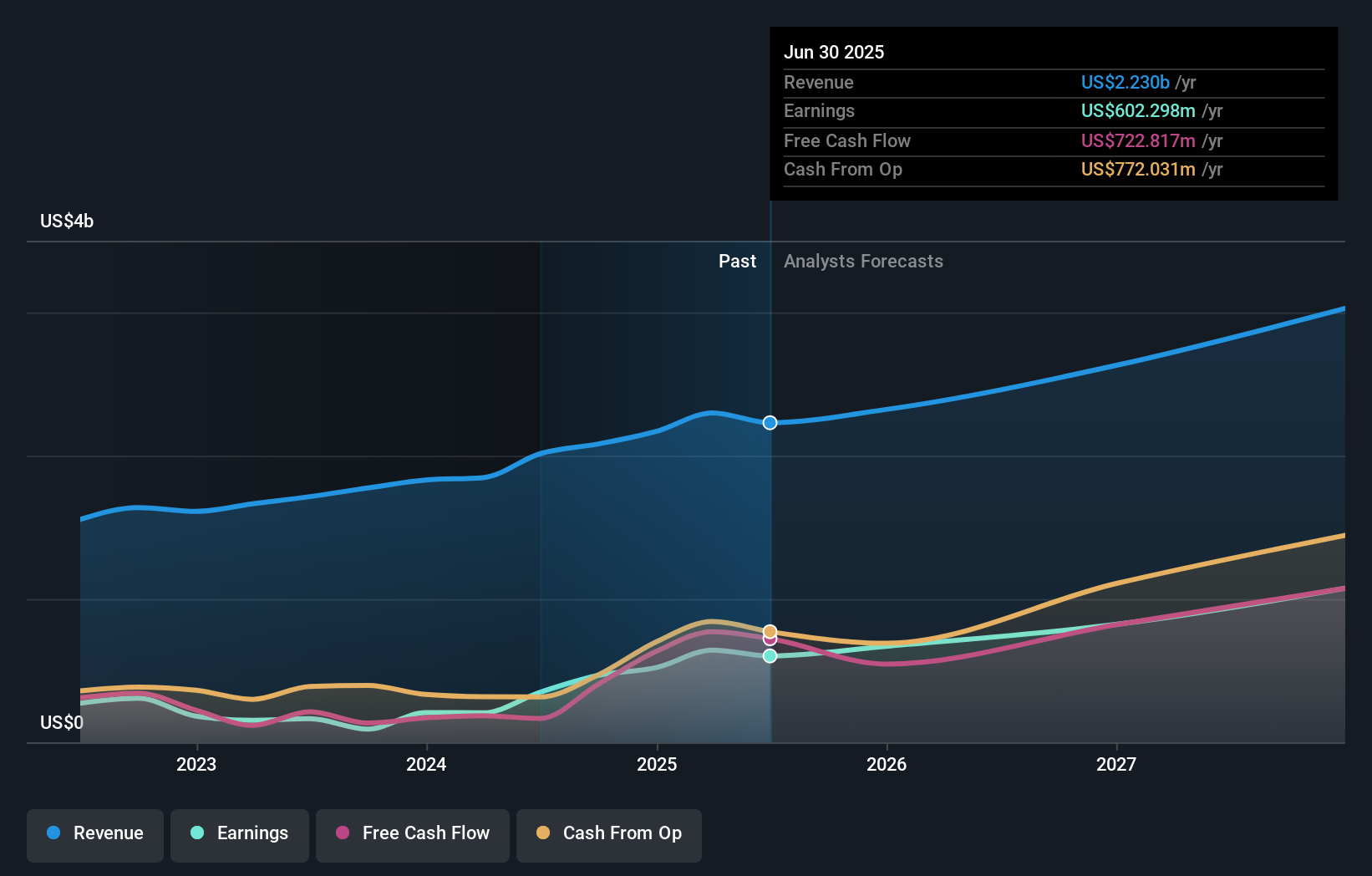
Exelixis, Inc. EXEL | 43.63 | -1.20% |
- FDA accepts Exelixis' New Drug Application for zanzalintinib plus atezolizumab in metastatic colorectal cancer.
- The application is based on pivotal trial data for patients with advanced disease.
- This step moves the investigational combination closer to potential commercial use in the United States.
For investors watching NasdaqGS:EXEL, this FDA filing milestone is an important datapoint in the company’s oncology pipeline. Exelixis focuses on cancer therapies, and colorectal cancer is a large, high-need treatment area where additional options are often closely followed by clinicians and payers.
This NDA acceptance does not guarantee approval. It does, however, start a formal regulatory review process that investors tend to track through upcoming FDA milestones. As timelines and any additional data updates emerge, they can affect how the market views Exelixis’ pipeline value and its potential role in metastatic colorectal cancer treatment.
Stay updated on the most important news stories for Exelixis by adding it to your watchlist or portfolio. Alternatively, explore our Community to discover new perspectives on Exelixis.
The FDA’s acceptance of Exelixis’ NDA for the zanzalintinib plus atezolizumab combination in previously treated metastatic colorectal cancer moves this asset into a clearly defined review timetable, with a PDUFA action date set for December 3, 2026. For you as an investor, the key point is that this filing is based on a head to head phase 3 trial versus regorafenib in a difficult later line setting. This positions Exelixis directly against existing options from players like Bayer, while also extending its footprint alongside large immuno-oncology peers such as Merck and Bristol Myers Squibb.
How This Fits Into The Exelixis Narrative
This news ties directly into the existing narratives that highlight Exelixis’ effort to reduce reliance on cabozantinib by building a broader oncology portfolio. Zanzalintinib, already being studied across several solid tumors, sits at the center of those views. An accepted NDA in colorectal cancer may be seen as one of the first real tests of whether that pipeline can support the kind of diversified, oncology-focused business profile many analysts have been discussing.
Risks And Rewards Investors Should Weigh
- 🎁 A potential new colorectal cancer indication could open up an additional revenue stream in a large treatment setting if the combination secures approval and achieves meaningful adoption.
- 🎁 Positive overall survival data versus regorafenib in the intention to treat population gives Exelixis a concrete clinical result to point to as it talks to oncologists and payers.
- ⚠️ Overall survival data in patients without active liver metastases are still immature, so there is residual clinical and regulatory uncertainty until the planned final analysis in mid 2026.
- ⚠️ Concentration risk remains, because if future readouts or the FDA review do not align with expectations, sentiment around zanzalintinib could swing and affect how investors view the broader Exelixis story.
What To Watch Next
From here, the milestones to keep on your radar are the final OS analysis in the non liver metastases subgroup, any safety or efficacy updates from regulators, and how oncologists react to the full STELLAR 303 data in practice as they compare it with other later line options. If you want to see how different investors are thinking about these moving parts, take some time to read the community views and analyst narratives for Exelixis in the company’s community narratives hub and decide which assumptions line up best with your own outlook.
This article by Simply Wall St is general in nature. We provide commentary based on historical data and analyst forecasts only using an unbiased methodology and our articles are not intended to be financial advice. It does not constitute a recommendation to buy or sell any stock, and does not take account of your objectives, or your financial situation. We aim to bring you long-term focused analysis driven by fundamental data. Note that our analysis may not factor in the latest price-sensitive company announcements or qualitative material. Simply Wall St has no position in any stocks mentioned.



