Please use a PC Browser to access Register-Tadawul
Summit Therapeutics Enters FDA Review As Ivonescimab Trials Broaden Potential
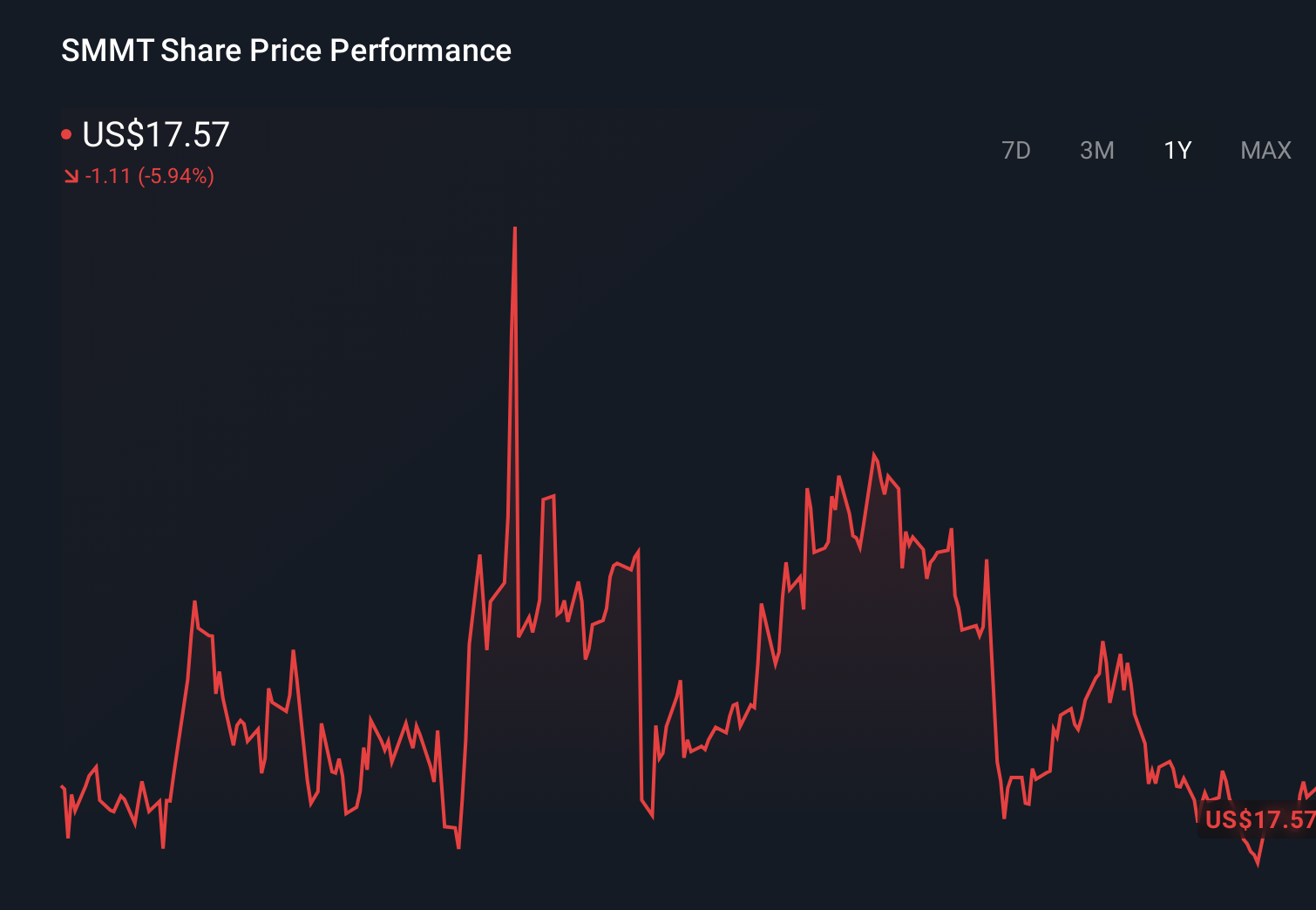
Summit Therapeutics Inc SMMT | 15.94 | +0.44% |
- Summit Therapeutics (NasdaqGM:SMMT) had its Biologics License Application for ivonescimab, in combination with chemotherapy, accepted by the US FDA for EGFR mutated advanced non squamous non small cell lung cancer after TKI therapy.
- The company is running several late stage clinical trials, and recent international Phase III results support expanding ivonescimab development beyond lung cancer.
- Ivonescimab is also being studied in colorectal cancer, reflecting broader pipeline ambitions across multiple tumor types.
For you as an investor, this positions Summit Therapeutics as a clinical stage oncology company with a focus on antibody based treatments for difficult to treat cancers. The FDA acceptance of a BLA is a formal step into the US regulatory review process, and together with multiple Phase III programs, it puts more attention on the company’s late stage pipeline rather than only early research.
Looking ahead, the key questions revolve around regulatory outcomes, how broadly ivonescimab might be used across indications, and what eventual commercial partnerships or infrastructure could look like if approvals are granted. As the trials progress, the timing and nature of future readouts and regulatory decisions will likely be important catalysts for anyone tracking NasdaqGM:SMMT.
Stay updated on the most important news stories for Summit Therapeutics by adding it to your watchlist or portfolio. Alternatively, explore our Community to discover new perspectives on Summit Therapeutics.
The FDA’s acceptance of Summit Therapeutics’ BLA for ivonescimab starts a clearly defined regulatory timeline, with a PDUFA goal date of 14 November 2026, which gives investors a firm reference point for when a US approval decision could be made. Because the filing is backed by Phase III HARMONi data in a specific post-tyrosine-kinase-inhibitor NSCLC setting, it also helps frame ivonescimab’s initial commercial focus in a crowded oncology space that includes players like Merck and Bristol Myers Squibb.
Summit Therapeutics narrative, now centered on late stage execution
This news shifts the Summit Therapeutics story more toward late stage regulatory and commercialization execution, rather than early development risk. With additional Phase III programs ongoing across NSCLC and colorectal cancer, the company narrative increasingly hinges on how consistently it can translate trial outcomes into regulatory filings across multiple tumor types.
Balancing risks and rewards from this regulatory step
- A filed BLA with a defined PDUFA date provides clearer visibility on timing for a potential first US product decision.
- Multiple Phase III trials across NSCLC and colorectal cancer create several potential value inflection points tied to clinical and regulatory outcomes.
- Summit currently generates less than US$1m in revenue, so the business remains highly dependent on external funding until any product is approved and commercialized.
- The company is currently unprofitable and not forecast to achieve profitability over the next 3 years, which heightens sensitivity to trial setbacks or regulatory delays.
What to watch next
From here, the key milestones to watch are interim and final readouts from the broader HARMONi program, any regulatory updates before the November 2026 PDUFA date, and signs of how Summit plans to handle commercialization in a market that already features treatments from Merck and Bristol Myers Squibb. If you want to see how other investors and analysts are thinking about these moving parts, you can check community narratives on Summit Therapeutics’ dedicated page.
This article by Simply Wall St is general in nature. We provide commentary based on historical data and analyst forecasts only using an unbiased methodology and our articles are not intended to be financial advice. It does not constitute a recommendation to buy or sell any stock, and does not take account of your objectives, or your financial situation. We aim to bring you long-term focused analysis driven by fundamental data. Note that our analysis may not factor in the latest price-sensitive company announcements or qualitative material. Simply Wall St has no position in any stocks mentioned.



